WGU C190 OA Study Guide II - 2025 | Mastering Meiosis, Mitosis, and Chemical Bonds: A Deep Dive into Biology 📖
Have you ever wondered how your body knows when to grow, heal, or even create the next generation? It’s all thanks to two essential processes—
This article explores two fundamental biological concepts that are essential for understanding cell processes and molecular interactions:
- Meiosis & Mitosis: These are two types of cell division that play crucial roles in growth, development, and reproduction. Mitosis produces identical cells for growth and repair, while meiosis creates genetically diverse gametes for reproduction.
- Chemical Bonds: Atoms combine to form molecules through different types of chemical bonds, including ionic, covalent, and hydrogen bonds. These bonds determine the structure and properties of substances, shaping everything from water molecules to complex biological compounds.
Students needing to answer WGU C190 OA questions should start by mastering fundamental biological and chemical principles that will help them succeed. These concepts need explanation in simple steps to help you study with assurance. Atoms show a preference for being in pairs because they naturally seek out connections to form larger compounds. The formation of chemical bonds enables the existence of molecules cells as well as all bodies in the universe.
How to Use This Guide for the WGU C190 OA Exam?📖
The C190 Introduction to Biology OA exam at WGU evaluates your understanding of biological processes, cellular functions, and the fundamental principles of biology. This guide simplifies the key concepts of meiosis & mitosis and chemical bonds to help you grasp the topics tested in the exam.
We also provide exam-style questions and practical applications to ensure you’re fully prepared for the questions on the WGU C190 OA exam.

Understanding Meiosis & Mitosis: The Fundamental Processes of Cell Division For C190 OA📝
Life’s basic units called cells remain active rather than staying idle. A living organism functions as a whole being because cells grow while they divide to execute vital processes that maintain organism survival. The biological process of cell division stands as one of the most significant because it supports growth in organisms alongside healing and reproduction functions. Cell division contains two main processes with different functions: mitosis and meiosis both names might share similarities in pronunciation.
If you’re preparing for your WGU C190 OA questions, understanding these processes will give you a strong foundation in biology. Let’s break them down step by step.
What is Mitosis?
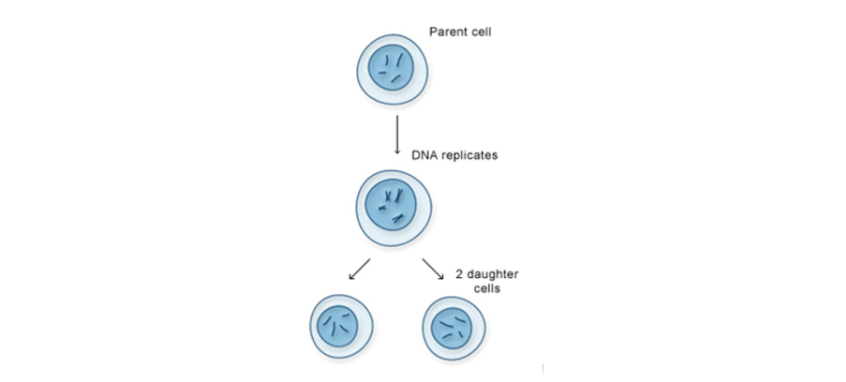
Mitosis is like making a photocopy of a document—you start with one cell, and it creates two identical copies. This type of cell division is crucial for growth, tissue repair, and asexual reproduction.
Stages of Mitosis
Mitosis happens in a sequence of steps to ensure that each new cell gets an exact copy of genetic material. These steps are:
- Prophase – The chromosomes condense and become visible under a microscope. The nuclear envelope starts breaking down, and spindle fibers begin to form.
- Metaphase – Chromosomes line up in the center of the cell, and spindle fibers attach to them.
- Anaphase – The sister chromatids (identical halves of a chromosome) separate and move to opposite ends of the cell.
- Telophase – The nuclear envelope re-forms around each new set of chromosomes, and the cell starts to split.
The cell achieves complete separation by means of cytokinesis which involves the cell membrane’s pinching action. These newly created cells possess genetic materials exactly equal to those of their source cell.
Each day at every moment of our lives our body executes mitotic processes. Human growth from one fertilized egg into a mature human being occurs through this process at the same time as wound healing and cellular maintenance.
What is Meiosis?
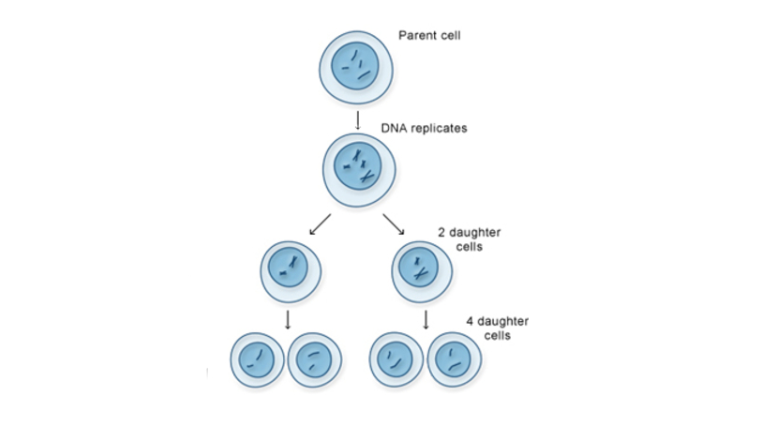
The only reproductive cells execute the specific cell division approach known as meiosis. The production method of meiosis differs from that of mitosis by yielding cells that have distinct genetic content from their parent cell. The cellular mechanism results in sperm and egg cells (gametes) to enable sexual reproduction.
Stages of Meiosis
Meiosis happens in two rounds of cell division instead of just one, leading to four daughter cells instead of two. These cells only have half the number of chromosomes of the original cell, which is important for sexual reproduction.
Meiosis I:
- Prophase I – Chromosomes condense and pair up with their matching partner. Crossing over happens, where genetic material is swapped between chromosomes, increasing genetic diversity.
- Metaphase I – Chromosome pairs line up randomly in the center of the cell. This randomness contributes to genetic variation.
- Anaphase I – The chromosome pairs separate and move to opposite sides of the cell.
- Telophase I – Two new cells form, but they still have double the necessary genetic material.
Meiosis II:
- Prophase II – Spindle fibers attach to chromosomes in the two new cells.
- Metaphase II – Chromosomes line up again at the center.
- Anaphase II – This time, sister chromatids separate and move to opposite ends.
- Telophase II – Four new cells form, each with half the number of chromosomes as the original cell.
These four cells are called haploid cells because they have only one complete set of chromosomes. When sperm and egg meet, they combine to form a diploid zygote, restoring the full number of chromosomes.
Key Differences Between Mitosis and Meiosis
Feature | Mitosis | Meiosis |
Purpose | Growth, repair, asexual reproduction | Sexual reproduction |
Number of Divisions | One | Two |
Number of Daughter Cells | Two | Four |
Genetic Variation | None (identical cells) | High (crossing over and random assortment) |
Chromosome Number | Stays the same (diploid) | Reduced by half (haploid) |
Where it Occurs | Somatic (body) cells | Reproductive cells |
Examples | Skin cell replacement, healing wounds | Formation of sperm and eggs |
Growth and repair processes utilize mitosis but meiosis serves the purpose of reproduction. Human growth and healing processes along with offspring genetic diversity production are impossible without both mitosis and meiosis operation.
Why Are Mitosis and Meiosis Important For C190 OA?
The two mechanisms serve dual purposes to preserve life because they support new skin cell growth following injuries and offspring production. Species succeed during evolutionary processes due to genetic diversity which meiosis establishes as a mechanism for species adaptation to environmental alterations.
For those studying WGU C190, mastering these concepts is essential. The differences between these two processes are common WGU C190 OA questions, so understanding the stages and functions of mitosis and meiosis will help you ace your assessments.
Conclusion
Life depends on the two essential processes of mitosis and meiosis to function. The human body grows and repairs itself through mitotic cell division but meiosis enables genetic adaptations for reproductive purposes.
Scientific knowledge about these processes does more than help you memorize science because it reveals to you how life preserves itself between generations. Your dedication to studying WGU C190 content will result in a comfortable mastery of all essential concepts!
Understanding Chemical Bonds: The Glue That Holds Matter Together For C190 OA📝
All things surrounding us such as air and food exist as particles known as atoms in the universe. Atomically speaking each atom chooses relationships rather than solitude because they thrive through the creation of bigger molecular units. Yet through chemical bonding, we possess molecules, and cells along with our entire physical existence!
For students preparing for their WGU C190 OA questions, understanding chemical bonds is essential. Let’s break them down step by step.
Types of Chemical Bonds
The process of attaining stability among atoms works through different mechanisms that involve electron transfers and sharing and electron attraction between atoms. The bonding process between atoms creates all the molecules which form the basis of our environment including both air and cellular structures present throughout our bodies. There are three main types of chemical bonds:
1. Ionic Bonds – The Electron Givers and Takers
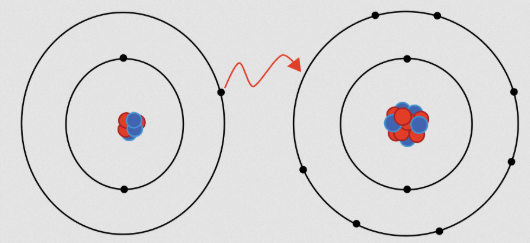
In ionic bonds, atoms trade election responsibility which makes one positively charged and the other negatively charged. The stable bond develops when oppositely charged ions attract strongly towards one another. The bonding process ensues when metals donate electrons to nonmetals which then receive these electrons.
How it Works:
- One atom loses electrons and becomes positively charged (a cation).
- Another atom gains electrons and becomes negatively charged (an anion).
- These opposite charges attract, forming a strong bond.
Example:
- Table salt (NaCl) – Sodium (Na) donates an electron to chlorine (Cl), forming a strong ionic bond.
Ionic bonds create solid structures like crystals and are often soluble in water. They also conduct electricity when dissolved, which is why saltwater can conduct electricity!
2. Covalent Bonds – The Electron Sharers
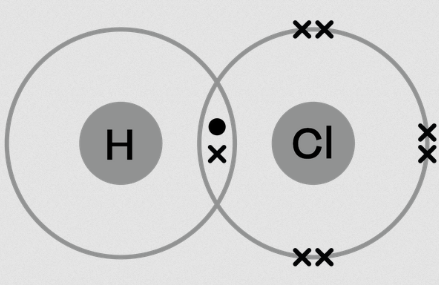
In covalent bonding, two nonmetallic elements share electrons as a method of bond formation. Strong covalent bonds enable the formation of molecules that constitute most living organisms. Covalent bonds establish stability by allowing atoms to complete their outer electron shell while nonmetal atoms exchange electrons instead of performing an ionic bond’s complete electron transfer. The atomic bond type depends on the number of electron pairs shared between nonmetals and it determines both substance structure and chemical properties.
How it Works:
- Two atoms share electrons to achieve a stable arrangement.
- The more electrons are shared, the stronger the bond. There are single, double, and triple covalent bonds.
Examples:
- Water (H₂O) – Oxygen shares electrons with two hydrogen atoms.
- Carbon dioxide (CO₂) – Carbon shares electrons with oxygen.
- Methane (CH₄) – Carbon shares electrons with four hydrogen atoms.
Covalent bonds can be polar or nonpolar:
- Polar covalent bonds – Unequal sharing of electrons, creating a molecule with slight charges (like water).
- Nonpolar covalent bonds – Equal sharing of electrons, creating a balanced molecule (like oxygen gas, O₂).
Covalent compounds tend to have lower melting points than ionic compounds and do not conduct electricity in water.
3. Hydrogen Bonds – The Weak Yet Important Bonds
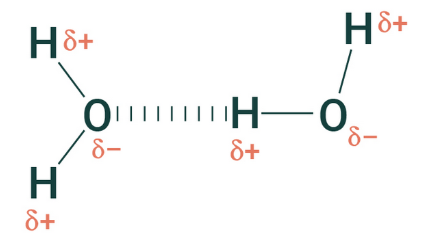
Biology greatly depends on weak hydrogen bonds because they function weaker than ionic or covalent bonds. The chemical connection happens when hydrogen atoms carrying a subtle positive charge become drawn to atoms with a negative charge like oxygen or nitrogen. The molecular bonds act as essential elements for biological operations since they preserve protein architecture and secure DNA architectures while enabling water to display its dual functioning abilities of tension strength and substance dissolution.
Examples:
- Water molecules – Hydrogen bonds give water its ability to form droplets and surface tension.
- DNA – The two strands of DNA are held together by hydrogen bonds.
- Proteins – Hydrogen bonds help shape proteins into their 3D structures.
Key Differences Between Ionic, Covalent, and Hydrogen Bonds
Property | Ionic Bonds | Covalent Bonds | Hydrogen Bonds |
Formation | Transfer of electrons | Sharing of electrons | Attraction between partial charges |
Strength | Strong | Strong | Weak |
Common Elements | Metals and nonmetals | Nonmetals | Hydrogen with oxygen, nitrogen, or fluorine |
Example | NaCl (Salt) | H₂O (Water) | DNA, Water Molecules |
Solubility in Water | High | Varies | High |
Conductivity | Conducts electricity in water | Does not conduct | Does not conduct |
Why Are Chemical Bonds Important For C190 OA?
Chemical bonds are responsible for the structure and function of all biological molecules. Here’s how they impact life:
- Ionic bonds help form minerals in bones and teeth.
- Covalent bonds hold together the molecules of life, such as proteins, DNA, and carbohydrates.
- Hydrogen bonds allow DNA to store genetic information and enable water to support life.
Chemical bonds also store energy. The energy our bodies use comes from breaking and forming chemical bonds, which is why food provides us with energy.
For those studying WGU C190, mastering these concepts is crucial. Understanding the differences between chemical bonds is key to answering WGU C190 OA questions effectively.
Conclusion
Bonds between chemicals function as unseen forces that maintain the structure of everything in existence. The existence of life depends on chemical bonds which also create salt in fries and structure DNA inside cells.
Knowledge of ionic, covalent, and hydrogen bonds grants you access to core concepts in chemistry and biology. Your exploration of WGU C190 key concepts will lead to confident mastery of this material soon!
Tired of reading blog articles?
Let’s Watch Our Free WGU C190 Practice Questions Video Below!

Wrapping Up: Conquering Mitosis, Meiosis, and Chemical Bonds for WGU C190 Success!📖
Learning about mitosis meiosis and chemical bonds brings more than classroom success because it demonstrates essential processes that sustain biological operations. Biology relies on cell division processes to grow along with the strength of chemical bonds that maintain molecular bonds in order to function.
For those gearing up for the WGU C190 OA, mastering these concepts is essential. Expect to see questions testing your knowledge of how cells divide and how atoms connect. So, take your time, review the material, and ensure you grasp the differences between mitosis and meiosis, as well as the nature of ionic, covalent, and hydrogen bonds.
Keep studying, stay curious, and best of luck on your WGU C190 OA! You’ve got this! From the salt on your fries to the DNA in your cells, these bonds make life possible.
By studying ionic covalent and hydrogen bonding mechanics you will become knowledgeable about chemical and biological principles. Your continued exploration of WGU C190 concepts will soon lead to a strong mastery of its important principles!